

To stabilise the nucleus, the protons starts converting into neutrons, positrons and a neutrino (which is 𝛽+ decay).įor 14O, it is a hybrid of 𝛽+ and electron capture. The loss of an electron by a lithium atom is more effective when it collides with a positron than with a proton or alpha particles. In positron emission, a proton inside the radioactive nucleus is converted into a neutron while releasing a positron in electron capture, a proton-rich nucleus of a neutral atom absorbs an inner shell electron which then converts a proton into a neutron, emitting an electron neutrino. That's were the weak nuclear force comes into play (which we will not discuss). The strong nuclear force doesn't depend upon the charge so as the number of protons increase and the electrostatic force starts dominating, and the nucleus needs more neutrons to stabilise. You can see that there is a exponential increase in the number of neutron and we increase the atomic number (Z).

To avoid this, you will see a pattern as the number of protons in a nucleus increases. This force has some properties which I will not specify, but you can read What is Nuclear Force? – Definition, Properties, Examples.īut as the number of protons in the nucleus increases the electrostatic force starts dominating over the strong nuclear force. This force is stronger than the electrostatic force. This is the force that glues protons and neutrons together. This is the most powerful force in nature (within suitable conditions). Mark, Geochronology and Thermochronology by the 40Ar/39Ar Method, 2nd Ed., Oxford, 1999. Freeman, 1969.Ģ.McDougall, Ian and Harrison, T. Brent and Lanphere, Marvin A., Potassium-Argon Dating, W.H. The energy-level diagram below is based on data accumulated by McDougall and Harrison 2.ġDalrymple, G. Similar can be found in The K/Ar dating method : principle, analytical techniques, and application to Holocene volcanic eruptions in Southern Italy:Įven though the decay of 40K is somewhat complex with the decay to 40Ca and three pathways to 40Ar, Dalrymple and Lanphere 1 point out that potassium-argon dating was being used to address significant geological problems by the mid 1950's. A direct simultaneous detection of electron (\(e-\)) and positron (\(e+\)) bunches was successfully performed using wideband beam monitors and a detection system at the \(e+\) capture section. Nonetheless here's a decay diagram from Hyperphysics that cites some historic work. It's hard to get the branching ratios exactly right because the decay by three different modes, some radiative, some to the ground state require different techniques to measure, and their efficiencies are difficult to normalize accurately. Very rarely (0.001% of events), it decays to 40Ar by emitting a positron (β+) and a neutrino The radioactive decay of this particular isotope explains the large abundance of argon (nearly 1%) in the Earth's atmosphere, as well as prevalence of 40Ar over other isotopes. In about 10.72% of events, it decays to argon-40 (40Ar) by electron capture (EC), with the emission of a neutrino and then a 1.460 MeV gamma ray.
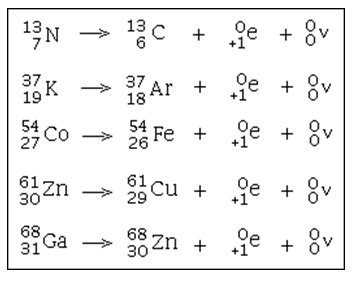
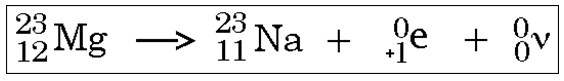
In about 89.28% of events, it decays to calcium-40 (40Ca) with emission of a beta particle (β−, an electron) with a maximum energy of 1.31 MeV and an antineutrino. Potassium-40 is a rare example of an isotope that undergoes both types of beta decay. This means that trails of these particles in a cloud chamber should be mirror reflections. An electron and positron have differing by a sign an identical charge with accuracy to twenty decimal places. Some isotopes can do both + and - beta decay, eg Cu-64. An electron and positron have identical mass with accuracy to many decimal places. Update!: comment allerts us of even more nuclear ambivalency or creativity: So nuclear wallet cards while incredibly handy aren't the last word in nuclear physics.
#Positron and electron capture plus


 0 kommentar(er)
0 kommentar(er)
